Fertile Male Dairy Cattle Fertile Male Beef Cattle
Abstract
Fertility plays a key role in the success of calf production, but there is evidence that reproductive efficiency in beef cattle has decreased during the past half-century worldwide. Therefore, identifying animals with superior fertility could significantly bear upon cow-calf production efficiency. The objective of this research was to place candidate regions affecting bull fertility in beef cattle and positional candidate genes annotated within these regions. A GWAS using a weighted single-step genomic BLUP arroyo was performed on 265 crossbred beefiness bulls to identify markers associated with scrotal circumference (SC) and sperm move (SM). 8 windows containing 32 positional candidate genes and five windows containing 28 positional candidate genes explained more than 1% of the genetic variance for SC and SM, respectively. These windows were selected to perform gene note, QTL enrichment, and functional analyses. Functional candidate gene prioritization analysis revealed 14 prioritized candidate genes for SC of which MAP3K1 and VIP were previously institute to play roles in male fertility. A different fix of 14 prioritized genes were identified for SM and five were previously identified every bit regulators of male fertility (SOD2, TCP1, PACRG, SPEF2, PRLR). Significant enrichment results were identified for fertility and body conformation QTLs within the candidate windows. Gene ontology enrichment assay including biological processes, molecular functions, and cellular components revealed significant Get terms associated with male person fertility. The identification of these regions contributes to a better understanding of fertility associated traits and facilitates the discovery of positional candidate genes for future investigation of causal mutations and their implications.
Introduction
As the human population continues to abound exponentially, so does the demand for animal proteins, such as beefiness and beef past-products1. In 2014, the Intergovernmental Panel on Climate Change (IPCC) report estimated that by 2050 meat consumption will rise 21% per capita every bit a result of a larger human being population, leading to business and economic benefits for beef producers2. One way we can continue to match the need of beef and beef by-products with the ever-growing population is through increasing beef cattle fertility3,4. This tin can be attained through the utilise of genetics to select for more fertile bulls, thereby increasing convenance success rates and decreasing the number of replacement heifers. However, over the last few decades, fertility in beef cattle has decreased because of intense option force per unit area on production traits3. 1 of the reasons for this subtract in fertility results from the pleiotropic issue of genes and biological processes underlying fertility and product, such as cell proliferation and energy conservation metabolism5,6. Therefore, farther inquiry on the functional biology underlying fertility traits in cattle is required to prevent these undesirable side effects of selection6,vii,8,9.
Genetic studies accept focused primarily on female fertility, which presents a pregnant challenge to improving beef cattle fertility because of the low heritability (0.01–0.x) of these reproductive traits10. Fertility-related traits in bulls are moderately heritable (0.05–0.22) and significantly influenced by genetics11. Therefore, improving our agreement of the genes and epigenetic modifications that contribute to bull fertility could meliorate reproduction success in beef cattle11,12. Indeed, sires direct influence the fertilization procedure, the viability of the preimplantation embryo, too as the conception charge per unit13. Sperm motility (SM) could be used as a proxy for identifying more fertile sires and improving reproduction success because information technology is a moderate to highly heritable trait (0.29–0.60) with significant components in the genome contributing to semen quality14,15,16. With reduced movement in bull semen becoming a prominent concern in the convenance industry, it is essential to go on studying genomic regions, variants and functional genes that impact motility15.
Scrotal circumference (SC) is also a practiced indicator trait for sire fertility because it is highly correlated with testes weight, sperm output, and semen quality17. Previous studies take suggested that a smaller SC is associated with lower fertility measures compared to bulls with a larger SC18. Moreover, a positive association has been reported between SC and the pct of live sperm, sperm number, and SM18. Therefore, the identification of genetic markers linked to SC and SM could be used as a strategy to genetically improve reproductive performance17. Even so, in that location is a negative genetic correlation between functioning traits, such as feed efficiency, and SC, with more feed-efficient bulls having smaller SC17,19. Thus, conscientious consideration must be taken into business relationship when selecting for both product and fertility traits to avoid undesirable effects.
Relatively fewer GWAS for bull fertility traits accept focused on crossbred beefiness cattle, which stand for a significant proportion of the beef cattle population, compared to purebred cattle20,21. This is in part due to multiple beef breeds with each breed association carrying out a split up genetic evaluation using different methods to calculate expected progeny deviation and the correspondence of SNP effects22. In this study nosotros used a sample size of 265 bulls with both SC and SM measurements. Numerous other male fertility GWAS in cattle have obtained interesting results with a very similar sample sizexv,18,23,24,25,26,27. Therefore, the objectives of the present study were (1) to detect SNP windows significantly associated with SC and SM in crossbred beef bulls using a single-step approach, and (2) to identify positional candidate genes with boosted functional bear witness and their potential office in bull fertility. To the best of our knowledge, this is the first study that uses a GWAS to analyze both SC and SM measurements simultaneously, in Canadian crossbred beef cattle, bringing new considerations for the current phase of literature regarding male fertility traits in beef cattle.
Results
Scrotal circumference genome wide association study and QTL enrichment
Subsequently quality control, 379,591 markers remained for analysis based on a call charge per unit greater than 95% and a minor allele frequency greater than 5% with non-autosomal markers. The Manhattan plot in Fig. 1a shows the non-overlapping windows 1 Mb apart which explain the highest proportion of variance for SC. Eight windows explained more than i% of the genetic variance for SC which were located on BTA9, BTA10, BTA20, BTA24 and BTA29, and explained 13.19% of the total genetic variance (Table 1). Thirty-two positional candidate genes were identified inside these windows explaining more than one% of the genetic variance for SC. ToppGene prioritization analysis revealed 14 of the 32 positional candidate genes were prioritized for spermatic-related processes and the windows which these genes are mapped inside explained 9.76% of the total genetic variance for SC. The QTL notation revealed several reproduction QTLs, annotated within the coordinates of the windows explaining > one% of the total genetic variance for SC, account for 7.85% of the QTLs annotated in those regions (Fig. 2a). The QTL enrichment assay revealed 38 significant QTLs (FDR-corrected p value ≤ 0.05) on BTA9 and BTA10 annotated for traits related to exterior conformation (Table 2; Fig. 2b).
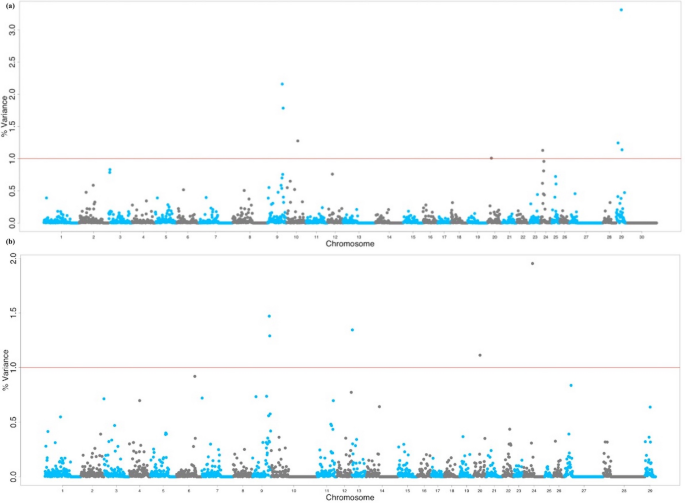
Manhattan plot for the GWAS of (a) scrotal circumference and (b) sperm motility in crossbred beef cattle. The y-axis shows the proportion of variance explained by the non-overlapping windows and the ten-axis indicates the chromosome number. The red line indicates the threshold for i% of the variance explained by the windows.
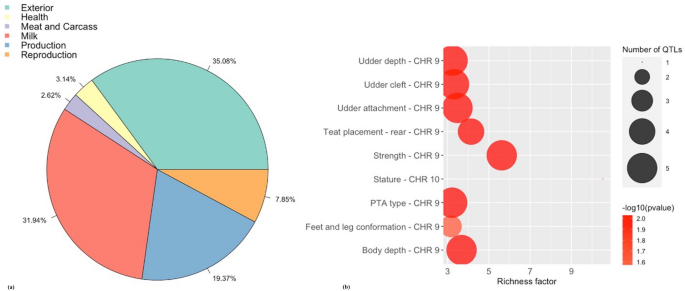
(a) Pie plot showing the percentage of each QTL class annotated in the windows explaining > one% of the total genetic variance for scrotal circumference. (b) Enriched traits identified in the QTL enrichment analysis for scrotal circumference. The area with the circles represents the number of observed QTLs for that class, while the color represents the p value calibration (the darker the colour, smaller the p value). The ten-centrality shows the richness factor for each QTL, representing the ratio of number of QTLs and the expected number of that QTL.
Sperm move genome broad association study and QTL enrichment
The Manhattan plot in Fig. 1b shows the non-overlapping SNP windows i Mb apart that explain the highest proportion of variance for SM. Five windows explained more 1% of the genetic variance for SM which were located on BTA9, BTA13, BTA20, and BTA24, and explained 7.17% of the total genetic variance (Table one). Twenty-eight positional candidate genes were identified within these SNP windows explaining more than one% of the genetic variance for SM. ToppGene prioritization revealed 14 of the 28 positional candidate genes were prioritized for spermatic-related processes and the windows which these genes are mapped within explained seven.16% of the total genetic variance for SM. The QTL note revealed several reproduction QTLs, annotated inside the coordinates of the windows explaining > 1% of the total genetic variance for SM, account for 8.eleven% of the QTLs annotated in these regions (Fig. 3a). The QTL enrichment analysis revealed 13 significant QTLs (FDR-corrected p value ≤ 0.05) on BTA9 and BTA13 annotated for traits related to reproduction and exterior conformation (Table 2; Fig. 3b).
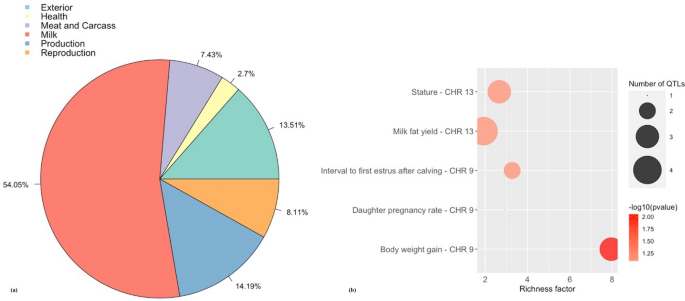
(a) Pie plot showing the percentage of each QTL class annotated in the windows explaining > 1% of the total genetic variance for sperm motility. (b) Enriched traits identified in the QTL enrichment assay for sperm motility. The area with the circles represents the number of observed QTLs for that form, while the color represents the p value calibration (the darker the color, smaller the p value). The x-axis shows the richness factor for each QTL, representing the ratio of number of QTLs and the expected number of that QTL.
Functional assay of prioritized candidate genes for scrotal circumference and sperm motility
The network constructed with the 14 prioritized candidate genes for SC and SM (full of 28 genes) using NetworkAnalyst had 1442 nodes (genes) related to 120 GO:BP (Table S2a), 78 Get:MF (Table S3a) and 69 GO:CC (Table S4a) terms. I hundred and twenty GO:BP were pregnant (FDR-corrected p value ≤ 0.05) and 64 were identified as related to male fertility and reproduction (Tabular array S2b). These 64 Become:BP terms were selected to construct the kickoff module containing 752 genes (Table S2c). A 2nd module was generated composed only past the prioritized candidate genes for SC and SM and its directly connected nodes leading to a network constructed of 20 genes and 26 Go:BP terms (Fig. 4a, Table S2d). None of the Become:BP in this module were significant (the smallest FDR-corrected p value was 0.128), notwithstanding nine were related to male fertility and reproduction of which regulation of MAPK pour, spermatid differentiation, and regulation of hormone secretion were the near notable (Table three). In this second module, five of the xx genes were prioritized candidate genes for SM and i gene was prioritized for SC.
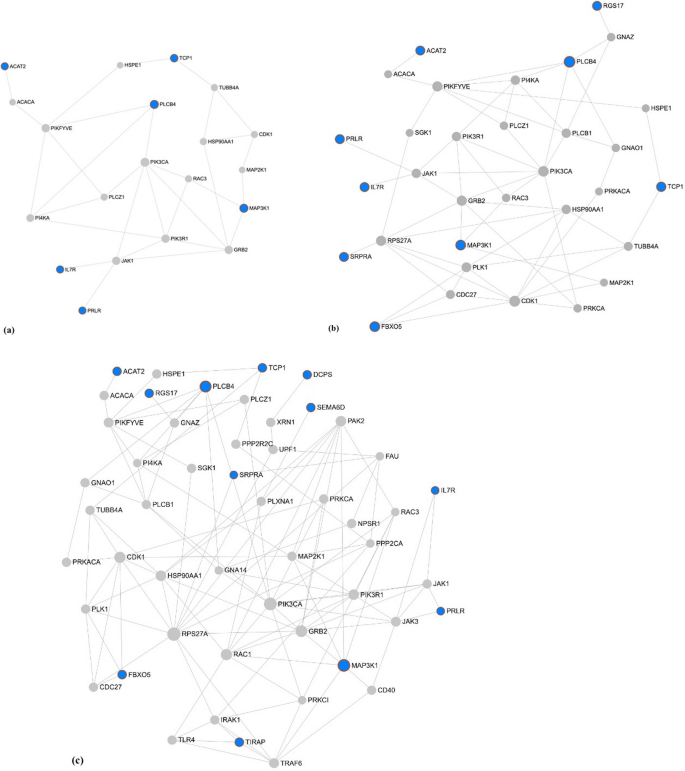
Cistron network generated in NetworkAnalyst iii.0 (https://www.networkanalyst.ca) via selection of the prioritized candidate genes for scrotal circumference and sperm motion and the significant (a) Biological process, (b) Molecular office, and (c) Cellular component GO terms involved in reproductive processes (FDR-corrected p value ≤ 0.05).The blue circles are the prioritized candidate genes for scrotal circumference and sperm move and the grey circles represent the directly connected genes.
Of the 78 Become:MF, 75 were significant (FDR-corrected p value ≤ 0.05) and 34 were identified as related to male fertility and reproduction functions (Table S3b). The start module for GO:MF terms was extracted using these 34 Go:MF terms containing 684 genes (Tabular array S3c). A second module for Go:MF was generated composed merely by the prioritized candidate genes for SC and SM and its directly continued nodes revealing a network constructed of 32 genes and 20 Go:MF terms (Fig. 4b, Table S3d). In this 2d module, 10 GO:MF were significant (FDR-corrected p value ≤ 0.05) and half-dozen of these were related to male fertility and reproduction including, acetyltransferase activity, zinc ion binding, lipase activeness, endonuclease activity, nuclease activity, and cation channel activity (Table iii). Five of the 32 genes in this second module for Get:MF were prioritized candidate genes for SM and 4 were prioritized candidate genes for SC.
Of the 69 Go:CC terms, 55 were significant (FDR-corrected p value ≤ 0.05) and 28 were related to male fertility and reproduction functions and processes (Table S4b). These 28 Go:CC terms were selected to extract the offset module for Go:CC which contained 992 genes (Table S4c). "Batch Selection" of the prioritized candidate genes for SC and SM was used to excerpt the second module for Become:CC which revealed a network containing 51 genes and 21 Become:CC terms (Fig. 4c, Table S4d). In this 2nd module, v Go:CC were significant (FDR-corrected p value ≤ 0.05) and three of these were related to male fertility and reproduction including, kinesin complex, spindle microtubule and cytosol (Tabular array 3). Five of the 51 genes in the 2nd GO:CC module were prioritized candidate genes for SM and seven genes were prioritized candidate genes for SC. A graphical representation of the functional analysis can be found in Fig. five.
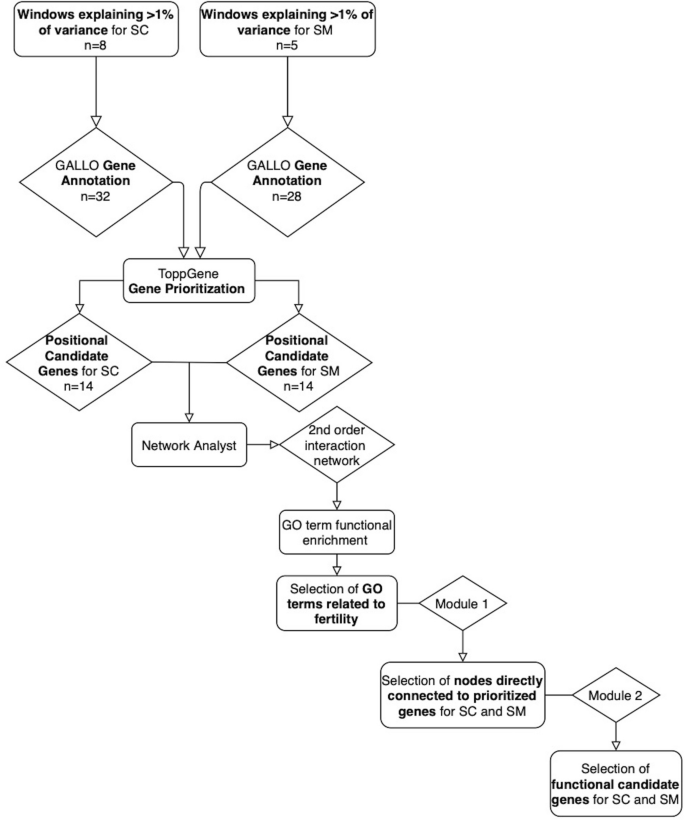
Graphical representation of the functional analyses performed to identify the positional candidate genes with additional functional prove for scrotal circumference and sperm motility.
Discussion
The ability to identify cattle with greater reproductive performance would significantly improve the efficiency of the beefiness cattle industry. Thus, the discovery of genetic markers related to male fertility through GWAS can contribute to the evaluation of fertility traits, such every bit SC and SM, and their implications on male person fertility. Spermatic related traits, including SM are normally measured in dairy cattle where semen is widely used for bogus insemination and measuring semen parameters is a standard practisexv,21,24,25,26,27. All the same, SM measurements are much less common in the beefiness industry where natural breeding is often used, which can be seen in our sample size. Testicular related traits, including SC have college heritability values and correlations with fertility traits, although the majority of studies on SC take been on purebred bulls18,21,28,29,30,31,32. Few SC GWAS take been conducted in crossbred beef cattle26,33, which represents a meaning proportion of the beefiness industry20. To the all-time of our knowledge, this is the first study that uses a GWAS to analyze both SC and SM measurements, simultaneously in Canadian crossbred beef cattle. The sample size in the nowadays study (265) might outcome in a careful interpretation of the obtained results, however many other GWAS have obtained notable results with similar sample sizes, ranging from 41 to 69215,18,23,24,25,26,27. For case, Hering et al.15 conducted a GWAS on 41 bulls with very poor SM and 279 bulls with first-class SM and identified nine candidate genes all with a strong relationship to sperm function. Buzanskas et al.26 . used SC records on 392 bulls in a GWAS and identified the STATU2 gene which participates in multiple biological processes, including reproduction, developmental and immune systems. When taken together, the limited studies conducted in crossbred beef cattle, the high heritability of SC and SM, and the evaluation of these traits together results in a study that can bring new considerations for the current stage of the literature regarding male person fertility traits in beef cattle.
The ssGBLUP method is based on an infinitesimal model and thus assumes equal variance for all SNP effects, posing an unrealistic situation for traits of economic interest, such as fertility34. Therefore, a weighted ssGBLUP (WssGBLUP) approached was used that combines pedigree, phenotype, and genotype data with the integration of different weights for markers in an iterative process to update SNP solutions. For SC, 8 windows, located on BTA9, BTA10, BTA24, and BTA29, explained more than than 13.19% of the total genetic variance. For SM, 5 windows explained more than 7.17% of the genetic variance and were located on BTA9, BTA13, BTA20, and BTA24. Of these, BTA9 explained the highest proportion of the variance for SC (4.03%) and SM (2.76%), respectively. Chromosome-wise and genome-wise associations have previously been identified in BTA9 for SC at 420 days in Canchim beef cattle26. Moreover, a region on BTA9 in Nellore cattle was found to be associated with testicular hypoplasia, which is defined by a reduced size of both testicles, and consequently reduced scrotal circumference and sperm physiology35.
Of the xiv prioritized candidate genes identified for SC (SASH1, VIP, FBX05, MTRF1L, RGS17, SLC24A5, SEMA6D, MAP3K1, SRPRA, TIRAP, DCPS, ST3GAL4, KIRREL3, CDON), ii, MAP3K1 and VIP , were previously reported equally related to male person reproduction. Mitogen-activated protein kinase kinase kinase i (MAP3K1), was mapped within a window that explained ane% of the variance in SM (BTA20: 22.18–23.18 Mb) and was of involvement for its anti- and pro-apoptotic functions in germ cells36. Guan et al.37 found the MAP3K1 gene was differentially expressed in testis from underfed and well-fed sexually mature sheep, indicating information technology could be a mark of germ jail cell apoptosis and therefore change SC and the efficiency of sperm production. The vasoactive intestinal peptide (VIP; BTA9: 89.51–90.51 Mb window), another prioritized candidate gene mapped within a window for SC that explained 1.61% of the variance, is known to act directly on the testis, promoting the production of testosterone in mice and rats38,39. In a report conducted by Lacombe et al. 39, VIP nada male mice exhibited a reduction in circulating concentrations of testosterone and follicle stimulating hormone (FSH), inhibiting the morphology of testicular seminiferous tubules. This gene has besides been found to play a role in mammalian folliculogenesis, ovarian evolution, and pubertyv,40,41.
Five of the prioritized candidate genes for SM have besides been previously identified as candidate genes in the regulation of male person fertility. These include, superoxide dismutase two, T-circuitous poly peptide 1, parkin co-regulated gene, sperm flagella two factor, and prolactin receptor (SOD2, TCP1, PACRG, SPEF2, PRLR). Ane of the master causes of sperm chromatin impairment is through oxidative stress caused past an imbalance between reactive oxygen species and scavenger systems42. Superoxide dismutase isoenzymes, like SOD2 (BTA9: 95.18–96.18 Mb window), destroy these toxic superoxide radicals that are ordinarily produced inside cells43. They have been constitute to be highly expressed in mammalian semen and their activity is positively associated with sperm concentration and move44,45. Another gene found to exist associated with spermatogenesis is TCP1 (BTA9: 95.18–96.eighteen Mb window), a member of the cytosolic chaperonin-containing TCP1 complex46, which has been identified in the cytosolic fraction47 and plasma membrane 48 of bovine spermatozoa. The PACRG and SPEF2 genes are essential for the development of normal sperm and male fertility. Previous studies involving PACRG (BTA9: 97.25–98.24 Mb window) knockdown in mice accept discovered it plays a vital role in maintaining the functional stability of flagella indicating an important relation to sperm move49,l. Moreover, Guo et al. 51 discovered SPEF2 (BTA20: 38.08–39.08 window) gene expression in the testes and sperm is regulated past culling splicing and is thus, one of the determining factors of sperm movement. Lastly, PRLR (BTA20: 38.08–39.08 window) gene expression was identified in the bovine reproductive tract including, the testis, epididymis, spermatogonia, and differentiating germ cells, leading researchers to believe it may accept an upshot on spermatogenesis52. This gene was also establish to play a part in fertilization and survival rates through SNP-SNP interactions53.
5 of the prioritized candidate genes for SC and SM have also been identified as playing important roles in the regulation of female fertility. Such genes include, semaphorin 6D, SRP receptor alpha subunit, Kirre like nephrin family unit adhesion molecule three, acetyl-CoA Acetyltransferase 2, and proto-oncogene, K protein-coupled receptor (SEMA6D, SRPRA, KIRREL3, ACAT2, MAS1). Both SEMA6D (BTA10: 22.x–63.21 Mb) and SRPRA (BTA29: 29.35–30.35 Mb window) are expressed in the human female genital tract, with SEMA6D linked to known gonadal genes54 and SRPRA upregulated during pregnancy and involved in the transport of secretory and membrane proteins55. On the other mitt, the KIRREL3 (BTA29: 29.35–30.35 Mb window), ACAT2 (BTA9: 95.eighteen–96.eighteen Mb window), and MAS1 (BTA9: 95.xviii–96.18 Mb window) genes take been previously studied in bovine. The KIRREL3 gene was institute to be highly expressed in granulosa cells and may act as a metabolic messenger linking metabolism, body limerick and fertility56. The ACAT2 gene was associated with both daughter pregnancy charge per unit and cow conception rate57, and MAS1 gene has been suggested to play a office in the regulation of ovulation58. Therefore, despite these genes being identified equally prioritized candidate genes regulating male fertility traits, SC and SM, they also play a office in female person fertility and regulation.
Through functional analysis, three modules were created with the prioritized candidate genes for SC and SM for the Get:BP, GO:MF, and GO:CC terms related to male fertility and reproduction. Even though nine Become:BP in the module (created via option of the Go terms related to reproductive processes and the prioritized candidate genes for SC and SM) were related to male fertility and reproduction, due to the few genes contained in the network, only one or ii genes were associated with these BPs, thus, none of the terms were significantly enriched. Despite this, it is worthy to highlight the most notable BPs in this network: regulation of MAPK cascade, spermatid differentiation, and regulation of hormone secretion. The MAPK cascade regulates spermatogenesis, sperm maturation, and the acrosome reaction, thereby playing an important function in male reproductive processes59. 1 of the genes involved in the regulation of MAPK cascade is the T-complex protein ane subunit alpha, TCP1, a prioritized candidate cistron for SM and is involved in the assembly of actin and tubulin filaments60. Actin filaments are nowadays in mammalian germ cells and are involved in a number of changes that occur during spermatogenesis such equally, the determination of jail cell shape, movement, maturation of spermatozoa, and capacitation61. In particular, actin proteins change their distribution in the sperm head during maturation and command the residue between globular actin and fibrous actin61. Another Get:BP, spermatid differentiation, is also an important part of spermatogenesis that involves the differentiation of spermatids into mature spermatozoa in the seminiferous tubules62. The prolactin receptor, a SM prioritized candidate factor, was found to be directly linked with spermatid differentiation. Moreover, spermatogenesis and reproductive success is completely dependent on the secretion of various hormones, including FSH, androgen, and testosterone. For example, testosterone levels and SC in bulls are known to be correlated, with peak testosterone levels being lower in bulls with smaller SC63. Six of the significant GO:MF were related to male fertility and reproduction, including acetyltransferase activeness, zinc ion binding, lipase activity, endonuclease activity, nuclease activity, and cation channel activity. Acetyltransferases, specifically, histone acetyltransferases, human action during spermatogenesis through the differentiation of spermatocytes64. Zinc is incorporated into spermatozoa where it is bound by seminal fluid Zn-interacting proteins and plays a protective role for sperm chromatin decondensation and sperm motility65. Moreover, a number of cation channels, including potassium and chloride channels, are involved in sperm motility, maturation, and the acrosome reaction66. 3 of the pregnant GO:CC terms were related to male fertility and reproduction, including, kinesin complex, spindle microtubule and cytosol. A number of kinesin families play fundamental roles in mammalian spermatogenesis, including mitosis, meiosis, acrosome biogenesis, and tail formation67. Moreover, kinesins, specifically kinesin-thirteen southward, regulate the spindle microtubule dynamics and control spindle assembly and kinetochore-microtubule attachments67. 1 example of the importance of the cytosol in male fertility is the cytosolic fraction of bovine spermatozoa, which exhibits tyrosine kinase activity associated with sperm capacitation and acrosome reaction47. The GO:BP term spindle microtubule contained the TIRAP and RGS17 genes which are prioritized candidate genes for SC and PRLR, a prioritized candidate gene for SM, indicating that these genes could be important biomarkers of bovine fertility.
The QTL note revealed reproduction QTLs business relationship for 7.85% and 8.11% of the QTLs annotated in the windows explaining > 1% of the total genetic variance for SC and SM, respectively. These reproduction QTLs included, reproductive efficiency, age at first calving, calving ease, daughter pregnancy rate, interval from first to last insemination, fertility index, conception charge per unit, and interval to first estrus after calving. The largest proportion of QTLs in this written report were related to milk production for both SC and SM (31.94% and 54.05%, respectively). A QTL enrichment analysis was conducted every bit the unproblematic bias of investigation in the QTL database for cattle can event in a larger proportion of records in the database. The enrichment analysis for the windows explaining > i% of the total genetic variance for SC and SM revealed QTLs annotated for outside conformation traits, including v unlike body depth, three unlike feet and leg conformation, five different force, and v different stature QTLs annotated inside the candidate windows for SC, and three dissimilar stature QTLs and three different body weight gain QTLs annotated within the candidate windows for SM (Table 2; Tabular array S5). In a study conducted by Schenkel et al. 68, SC was found to exist genetically correlated (P < 0.05) to average daily gain, ultrasound backfat thickness, mid-test metabolic weight and hip tiptop in 13,151 bulls (r g = 0.24, 0.19, 0.31, 0.16, respectively). Some other study revealed a positive correlation for SC and body weight in both pubertal and mail service-pubertal Holstein bulls (r thousand = 0.76 and 0.45, respectively69). Therefore, bulls with larger SC have a larger torso size and faster growth68,69,lxx. This suggests that these regions may exist regulating both fertility and conformation traits, however the biological mechanisms associated with this correlation is not well understood6,71,72. Other enriched QTLs for SC included udder depth, udder attachment, teat placement-rear, and udder cleft (Tabular array 2; Table S5). Cows with tightly attached udders and proper teats tend to remain in a herd longer, are easier to nurse, and less susceptible to mastitis, therefore udder traits may be used in conjunction with body conformation traits for the indirect pick of longevity in beef cattle73,74. The QTL enrichment analysis for SM too revealed QTLs related to fertility on BTA9, one for daughter pregnancy rate and two dissimilar QTLs for interval to starting time rut later on calving (Tabular array ii; Tabular array S5). This is consistent with other studies that reveal a genetic correlation between male and female fertility traits. For example, Johnston et al. 75 found SM at xviii months of historic period to be highly correlated with female person reproduction traits in Brahman and Tropical Composite cows, such as conception (0.53 and 0.72, respectively) and pregnancy rate (0.58 and 0.95, respectively). Gargantini et al. 76 identified a genetic correlation between yearling SC and historic period at puberty and pregnancy rate in heifers was − 0.57 and 0.35, respectively. Thus, BTA9 may exist a candidate region for bovine fertility. A study conducted by McClure et al. 77 identified 41 SC QTLs in Angus cattle, of which three QTLs were identified on BTA9. The overlap between the candidate regions identified in the present study and previous studies reinforces the association of these genomic regions with the regulation of genes and biological processes responsible for male fertility traits, including VIP and SOD2 genes, mapped on BTA9, and the regulation of MAPK pour and spermatid differentiation.
To conclude, functional assay for the prioritized candidate genes identified in this report revealed significant Go terms associated with biological processes and molecular functions related to male person fertility and reproduction. For scrotal circumference, both MAP3K1 and VIP identified genes control testis function and could be used every bit potential biomarkers of spermatogenesis and apoptosis. For sperm motility, the SOD2, TCP1, PACRG, SPEF2, and PRLR genes were related to sperm concentration, development, and motility. These results assistance to better understand the genetic bases of scrotal circumference and sperm motion specifically in crossbred beef bulls and revealed positional candidate genes with additional functional prove that might ultimately improve bull genomic prediction for these traits. Moreover, these candidate regions, specifically those mapped on BTA9, have known genetic correlations to other economically important traits including, conformation, female fertility, and udder construction. However, future research on these candidate genes and their impact on bull fertility is warranted.
Materials and methods
Population structure and phenotypic data
Data used in this report are from animals cared for nether protocols approved by the University of Guelph Brute Care Commission, which follows guidelines of the Canadian Quango on Animal Care (1993).
The population for this study came from the Ontario Beef Research Middle; the Academy of Guelph research farm located in Elora, ON, Canada and consisted of 265 crossbred bulls. At the time of drove, bulls had an boilerplate age of 384 days and average weight of 555 kg. The predominant breeds (and corresponding average composition of that brood in the test group, see Table S1) of these crossbred bulls were Angus (AN: 52%), Simmental (SM: 24%), Piedmontese (PI: half-dozen.six%), Gelbvieh (GV: six.3%), Charolais (CH: 3.8%), and Limousine (LM: one.ane%).
The fertility traits used in this study were SC and SM. Scrotal circumference was measured by palpating the testes into the lower half of the scrotum and measuring the greatest circumference using a looped record as described by Awada et al. 19. Semen ejaculates were nerveless between 12 and 15 months of historic period immediately after the SC measurement using electroejaculation (Pulsator IV- Auto Adjust Electro-Ejaculator: Lane Manufacturing, Inc, Denver, CO). Sperm motility was visually estimated immediately and ejaculates were and then extended, cooled, placed in liquid nitrogen and thawed as previously described by Pursel & Johnson78. Sperm motility was assessed every bit described by Awada et al. 79. Briefly, sperm were evaluated on the CASA (Integrated Visual Optical Organisation (IVOS) CASA System, Hamilton Thorne, Inc., Beverly, MA) sampled from one × 107 spermatozoa/ml in BTS78 and a four-sleeping room standard count analysis slide (Leja products B.V. Luzernestraat 10, 2153 GN Nieuw-Vennep, Kingdom of the netherlands), at 37 °C.
Genotyping and quality control
Genotyping was completed from 265 animals using the Affymetrix Genechip Bovine Genome High Density Array, which included 648,874 SNPs. Marker coordinates were converted to the new bovine genome associates ARS-UCD 1.2. Quality control was performed using Plinklxxx and the following criteria were used for the exclusion of SNPs: non-autosomal regions; minor allele frequency < 0.05; and a call rate < 95%.
Genome-wide association study
The programs of BLUPF90 family were used for the weighted single-step genomic BLUP (WssGBLUP) analysis81. The GWAS results were reported equally the proportion of the variance explained past non-overlapping genomic windows of ane Mb82.
A WssGBLUP method was used to estimate SNP furnishings. The observed phenotypes of SC and SM were used equally dependent variables in a single-trait creature model:
where y represented a vector of observed phenotypes for animals (SC or SM); X is the incidence matrix of fixed furnishings; b is the vector of fixed effects, Z a is the incidence matrix of additive genetic effects; a is the random vector of condiment genetic effects; and e is the vector of residual furnishings. Stock-still effects for SC included 25 levels of herd-year-flavour (HYS), torso weight, historic period every bit a third-degree polynomial, and breed composition for the nigh prevalent breeds (AN, SM, PI, GV, CH, and LM). Fixed effects for SM included 25 levels of HYS, age, and breed composition for the nearly prevalent breeds.
The solutions of the SNP effects (û) were obtained using the AIREMLF9081 algorithm with two iterations, as proposed by Wang et al. 34.
For each iteration of the algorithm D(n) = I and Thou(north) = ZD(due north) Z'λ, where D is a diagonal matrix of weights for SNP variances, G is the weighted genomic relationship matrix, Z is a matrix relating genotypes of each locus, λ is a variance ratio, and n is the iteration number; the breeding values (â v ) were calculated using single-stride genomic all-time linear unbiased predictor (ssGBLUP); SNP effects were calculated via û(n) = λD(due north)Z′G(due north) −1â five ; SNP weights were calculated via di(n+1) = û2 i(n)2p i (1 − p i ), where i is the ith SNP; the weights were normalized via D(northward+1) \({ } = \frac{{tr(D_{\left( 0 \correct)} )}}{{tr(D_{{\left( {north + 1} \right)}} )}}\) D(n+ane) so the additive genetic variance remains constant; and the Genomic matrix was recalculated via Yard(n+1) = ZD( n+i) Z'λ to obtain the SNP effects. These iterations were used to calculate the proportion of variance explained past not-overlapping windows.
The proportion of variance explained by non-overlapping windows were estimated using the PostGSf90 algorithm by summing the variance of SNPs within i Mb82. Windows that explained greater than one% of the genetic variance for SC and SM were selected for QTL and gene annotation83, conducted using R (Version 4.0.0.; R Cadre Team, 2020) and the R parcel: Genomic Annotation in Livestock for positional candidate LOci (GALLO—https://github.com/pablobio/GALLO). The .gtf annotation file respective to the bovine cistron notation from ARS-UCD1.ii assembly and the .gff file with the QTL information from Brute QTL Database84, using the same reference genome (ARS-UCD1.2) to map the QTLs, were used for cistron and QTL annotation, respectively. A QTL enrichment analysis was also conducted using the GALLO R package for all the QTL information annotated within the candidate windows using a chromosome-based enrichment analysis. Briefly, a bootstrap arroyo was used to compare the observed number of QTL for each trait in each chromosome annotated using GALLO with the expected number for each trait estimated using 1000 iteration rounds of random sampling from the whole Cattle QTLdb. Using this approach, a p value for the QTL enrichment status of each annotated trait within the candidate windows was calculated, Additionally, the p values were corrected for multiple testing using FDR (5%).
Cistron prioritization assay
Functional candidate gene prioritization was conducted using the ToppGene Suite85. A trained list of genes associated with keywords outlined past Fonseca et al. 21 using the GUILDify86 software and a species-specific (Man sapiens) interaction network, including, "scrotal circumference," "scrotal," "testicular," "testis," "testes," "sperm," "semen," "spermatozoa," "spermatogenesis," and "fertility," fabricated upwards the "trained list" of genes. From this analysis, the top 100 genes ranked using an algorithm based on network topology on GUILDify were used to create the "trained" cistron list. This "trained" gene list was used in conjunction with the "exam" gene list containing the genes within windows explaining greater than one% of the genetic variance for SC and SM. An annotation-based prioritization analysis through a multivariate approach was conducted using ToppGene. Gene Ontology terms for molecular function (MF), biological procedure (BP), and cellular component (CC); human and mouse phenotypes; metabolic pathways; Pubmed publications; and diseases were utilized to retrieve functional data for the trained list and for the list of positional candidate genes (those genes annotated within windows explaining more 1% of the genetic variance for the trait). Overall p values were obtained using a combination of the p values obtained from the intermediate values from the above functional information using a random sampling of 5000 genes from the whole genome for each notation information. Significant prioritized genes were selected based on a FDR v% multiple correction, pregnant these genes have a functional profile that is significantly similar to the functional profile of the "trained" gene list. As the "trained" gene list is known to be associated with fertility, by the guilty by association principle, it is likely that the prioritized genes will also be associated with fertility.
Functional analysis
A protein–protein interaction network analysis was performed in order to identify interactions between the positional candidate genes and other genes in the genome that are relevant to fertility. Therefore, the prioritized candidate genes for both SC and SM were inputted together into NetworkAnalyst 3.0 (87 https://www.networkanalyst.ca) and the STRING interactome poly peptide–poly peptide interaction database was used with a confidence score cutoff of 900. A 2nd-society interaction network was generated containing 1442 nodes (genes) with 4888 edges. Then, gene ontology (Go) enrichment analyses, including the three main categories biological procedure (BP), molecular functions (MF), and cellular components (CC), was performed using the genes comprising the network88. Functional evidence of the relationship between the meaning Get terms (FDR-corrected p value ≤ 0.05) and the target phenotypes (SC and SM) was identified. The GO terms related to reproductive processes were selected to extract the outset module from this network composed just by the nodes (genes) associated with the selected GO:BP, GO:MF and Become:CC. Next, a 2nd module was extracted, from the previous sub-network. This second module was composed only by the positional prioritized candidate genes for SC and SM and its straight connected nodes. The Become:BP, Go:MF and GO:CC enriched for this 2nd module were analyzed for its association with reproductive processes.
Abbreviations
- ssGBLUP:
-
Unmarried-stride genomic BLUP
- WssGBLUP:
-
Weighted single-step genomic BLUP
- SC:
-
Scrotal circumference
- SM:
-
Sperm movement
- FDR:
-
Simulated discovery rate
- AN:
-
Angus
- SM:
-
Simmental
- PI:
-
Piedmontese
- GV:
-
Gelbvieh
- CH:
-
Charolais
- LM:
-
Limousine
- HYS:
-
Herd-year-flavor
- GALLO:
-
Genomic Note in Livestock for positional candidate LOci
- MF:
-
Molecular role
- BP:
-
Biological procedure
- CC:
-
Cellular component
- MAP3K1:
-
Mitogen-activated protein kinase kinase kinase 1
- VIP:
-
Vasoactive intestinal peptide
- FSH:
-
Follicle stimulating hormone
- SOD2:
-
Superoxide dismutase 2
- TCP1:
-
T-complex protein ane
- PACRG:
-
Parkin co-regulated gene
- SPEF2:
-
Sperm flagella two factor
- PRLR:
-
Prolactin receptor
- SEMA6D:
-
Semaphorin 6D
- SRPRA:
-
SRP receptor alpha subunit
- KIRREL3:
-
Kirre similar nephrin family adhesion molecule 3
- ACAT2:
-
Acetyl-CoA Acetyltransferase ii
- MAS1:
-
Proto-oncogene, G poly peptide-coupled receptor
- TCP1:
-
T-complex poly peptide one subunit alpha
References
-
Cerri, C. C. et al. Assessing the carbon footprint of beef cattle in Brazil: a example study with 22 farms in the Land of Mato Grosso. J. Clean Prod. 112, 2593–2600. https://doi.org/10.1016/j.jclepro.2015.10.072 (2016).
-
Revell, B.J. One human being's meat...2050? Ruminations on time to come meat need in the context of global warming. J. Agric. Econ. 66, 573–614. https://doi.org/x.1111/1477-9552.12121 (2015).
-
Thundathil, J. C., Dance, A. L. & Kastelic, J. P. Fertility direction of bulls to improve beef cattle productivity. Theriogenology 86, 397–405. https://doi.org/x.1016/j.theriogenology.2016.04.054 (2016).
-
Nguyen, L. T. et al. STAT6, PBX2, and PBRM1 emerge as predicted regulators of 452 differentially expressed genes associated with puberty in Brahman heifers. Forepart. Genet. https://doi.org/ten.3389/fgene.2018.00087 (2018).
-
Cánovas, A. et al. Multi-tissue omics analyses reveal molecular regulatory networks for puberty in composite beefiness cattle. PLoS ONE https://doi.org/10.1371/journal.pone.0102551 (2014).
-
Fonseca, P. A. S. et al. Combining multi-OMICs information to identify key-regulator genes for pleiotropic effect on fertility and production traits in beef cattle. PLoS One https://doi.org/10.1371/journal.pone.0205295 (2018).
-
Rauw, Due west., Kanis, E., Noordhuizen-Stassen, E. & Grommers, F. Undesirable side effects of choice for high production efficiency in subcontract animals: a review. Livest. Prod. Sci. 56, 15–33. https://doi.org/10.1016/S0301-6226(98)00147-X (1998).
-
de Camargo, One thousand. M. et al. Not-synonymous mutations mapped to chromosome X associated with andrological and growth traits in beef cattle. BMC Genomics 16, 384. https://doi.org/10.1186/s12864-015-1595-0 (2015).
-
Dias, G. M. et al. SNP detection using RNA-sequences of candidate genes associated with puberty in cattle. Genet. Mol. Res. https://doi.org/x.4238/gmr16019522 (2017).
-
Ortega, M. S., Denicol, A. C., Cole, J. B., Nil, D. J. & Hansen, P. J. Use of unmarried nucleotide polymorphisms in candidate genes associated with daughter pregnancy rate for prediction of genetic merit for reproduction in Holstein cows. Anim. Genet. 47, 288–297. https://doi.org/10.1111/age.12420 (2016).
-
Berry, D. P., Wall, E. & Pryce, J. E. Genetics and genomics of reproductive functioning in dairy and beef cattle. Animal eight, 105–121. https://doi.org/x.1017/S1751731114000743 (2014).
-
Suchoocki, T. & Szyda, J. Genome-wide association study for semen production traits in Holstein-Friesian bulls. J. Dairy Sci. 98, 5774–5780. https://doi.org/10.3168/jds.2014-8951 (2015).
-
Han, Y. & Peñagaricano, F. Unravelling the genomic architecture of bull fertility in Holstein cattle. BMC Genet. 17, 143. https://doi.org/10.1186/s12863-016-0454-6 (2016)
-
Druet, T. et al. Estimation of genetic parameters and genome scan for 15 semen characteristics traits of Holstein bulls. J. Anim. Breed. Genet. 126, 269–277. https://doi.org/10.1111/j.1439-0388.2008.00788.x (2009).
-
Hering, D. One thousand., Oleński, Yard. & Kaminski, Due south. Genome-wide association study for poor sperm move in Holstein-Friesian bulls. Anim. Reprod. Sci. 146, three–4. https://doi.org/10.1016/j.anireprosci.2014.01.012 (2014).
-
Al-Kanaan, A., König, S. & Brügemann, K. Effects of estrus stress on semen characteristics of Holstein bulls estimated on a continuous phenotypic and genetic scale. Livest. Sci. 177, 15–24. https://doi.org/10.1016/j.livsci.2015.04.003 (2015).
-
Wang, Z. et al. Impact of selection for residue feed intake on breeding soundness and reproductive operation of bulls on pasture-based multisire mating. J. Anim. Sci. ninety, 2963–2969. https://doi.org/10.2527/jas.2011-4521 (2012).
-
Gipson, T. A., Vogt, D. W., Massey, J. W. & Ellersieck, M. R. Associations of scrotal circumference with semen traits in young beefiness bulls. Theriogenology 24, 217–225. https://doi.org/10.1016/0093-691X(85)90186-4 (1985).
-
Awda, B. J. et al. The human relationship between feed efficiency traits and fertility in young beefiness bulls. Can. J. Anim. Sci. 93, 185–192. https://doi.org/10.4141/cjas2012-092 (2012).
-
Lu, D. et al. Genome-wide association analyses for growth and feed efficiency traits in beef cattle. J. Anim. Sci. 91, 3612–3633. https://doi.org/10.2527/jas.2012-5716 (2013).
-
Fonseca, P. A. Southward. et al. Genetic mechanisms underlying spermatic and testicular traits inside and amid cattle breeds: systematic review and prioritization of GWAS results. J. Anim. Sci. 96, 4978–4999. https://doi.org/x.1093/jas/sky382 (2018).
-
Dalton, J.C. Semen quality factors associated with fertility. In Proceedings of Practical Reproductive Strategies in Beef Cattle—Northwest. 265–281 (2011).
-
Hering, D. Thousand., Oleński, M. & Kaminski, South. Genome-wide clan written report for sperm concentration in Holstein-Friesian bulls. Reprod. Domest. Anim. 49, 1008–1014. https://doi.org/10.1111/rda.12423 (2014).
-
Kamiński, S., Hering, D. K., Oleński, Chiliad., Lecewicz, One thousand. & Kordan, West. Genome-wide association report for sperm membrane integrity in frozen-thawed semen of Holstein-Friesian bulls. Anim. Reprod. Sci. 170, 135–140. https://doi.org/10.1016/j.anireprosci.2016.05.002 (2016).
-
Puglisi, R. et al. Genome wide assay for bull sperm quality and fertility traits. Reprod. Domest. Anim. 51, 840–843. https://doi.org/10.1111/rda.12747 (2016).
-
Buzanskas, M. Eastward. et al. Candidate genes for male and female reproductive traits in Canchim beef cattle. J. Anim. Sci. Biotechno. https://doi.org/10.1186/s40104-017-0199-viii (2017).
-
Qin, C. et al. Genome-broad association study for semen traits of the bulls in Chinese Holstein. Anim. Genet. 48, 80–84. https://doi.org/10.1111/historic period.12433 (2017).
-
Fortes, K. R., Reverter, A., Hawken, R. J., Bolormaa, S. & Lehnert, S. A. Candidate genes associated with testicular evolution, sperm quality, and hormone levels of inhibin, luteinizing hormone, and insulin-similar growth factor ane in Brahman bulls. Biol. Reprod. 87, 58. https://doi.org/x.1095/biolreprod.112.101089 (2012).
-
Utsunomiya, Y. T. et al. Genome-wide mapping of loci explaining variance in scrotal circumference in Nellore cattle. PLoS ONE 9, e88561. https://doi.org/10.1371/journal.pone.0088561 (2014).
-
Irano, N. et al. Genome-wide association study for indicator traits of sexual precocity in Nellore cattle. PLoS ONE 11, e0159502. https://doi.org/10.1371/journal.pone.0159502 (2016).
-
Soares, A. C. C. et al. Multiple-trait genomewide mapping and gene network analysis for scrotal circumference growth curves in Brahman cattle. J. Anim. Sci. 95, 3331–3345. https://doi.org/ten.2527/jas.2017.1409 (2017).
-
Menegassi, S. R. O. et al. Evaluation and prediction of scrotal circumference in beef bulls. Theriogenology 140, 25–32. https://doi.org/x.1016/j.theriogenology.2019.08.008 (2019).
-
Fortes, M. R. et al. Genomic regions associated with fertility traits in male and female cattle: advances from microsatellites to high-density chips and across. Anim. Reprod. Sci. 141, 1–19. https://doi.org/ten.1016/j.anireprosci.2013.07.002 (2013).
-
Wang, H., Misztal, I., Aguilar, I., Legarra, A. & Muir, W. M. Genome-wide association mapping including phenotypes from relatives without genotypes. Genet. Res. Camb. Core 94, 73–83. https://doi.org/10.1017/S0016672312000274 (2012).
-
Neves, H. H. R. et al. Genetic and genomic analysis of testicular hypoplasis in Nellore cattle. PLoS Ane https://doi.org/10.1371/journal.pone.0211159 (2019).
-
Pham, T. T., Angus, S. P. & Johnson, G. 50. MAP3K1: Genomic alterations in cancer and function in promoting cell survival or apoptosis. Genes Cancer 4, 419–426. https://doi.org/x.1177/1947601913513950 (2013).
-
Guan, Y., Liang, Thousand. & Martin, G. B. Functional changes in mRNA expression and alternative pre-mRNA splicing associated with furnishings of nutrition on apoptosis and spermatogenesis in the adult testis. BMC Genet. 18, 64. https://doi.org/10.1186/s12864-016-3385-8 (2017).
-
El-Gehani, F., Tena-Sempere, M. & Huhtaniemi, I. Vasoactive intestinal peptide stimulates testosterone production by cultured fetal rat testicular cells. Mol. Cell. Endocrinol. 140, 175–178. https://doi.org/10.1016/S0303-7207(98)00047-1 (1998).
-
Lacombe, A. et al. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J. Endocrinol. 194, 153–160. https://doi.org/10.1677/JOE-07-0102 (2007).
-
Gabbay-Benziv, R. et al. Vasoactive intestinal peptide and its receptors in human being ovarian cortical follicles. PLoS ONE https://doi.org/10.1371/journal.pone.0037015 (2012).
-
Chen, N. et al. Vasoactive intestinal peptide can promote the evolution of neonatal rat primordial follicles during in vitro culture. Biol. Reprod. 88, one–8. https://doi.org/x.1095/biolreprod.111.098335 (2013).
-
Simōes, R. et al. Influence of bovine sperm Dna fragmentation and oxidative stress on early on embryo in vitro evolution issue. Reprod. 146, 433–441. https://doi.org/10.1530/REP-xiii-0123 (2013).
-
Peeker, R., Abramsson, L. & Marklund, South. L. Superoxide dismutase isoenzymes in human seminal plasma and spermatozoa. Mol. Hum. Reprod. 3, 1061–1066. https://doi.org/x.1093/molehr/3.12.1061 (1997).
-
Yan, L. et al. Seminal superoxide dismutase activeness and its relationship with semen quality and SOD gene polymorphism. J. Aid. Reprod. Genetics 31, 549–554. https://doi.org/10.1007/s10815-014-0215-two (2014).
-
Yu, B. & Huang, Z. Variations in antioxidant genes and male infertility. BioMed Res. Int. https://doi.org/10.1155/2015/513196 (2015).
-
Lalancette, C., Thibault, C., Bachand, I., Caron, N. & Bissonnette, N. Transcriptome analysis of bull semen with farthermost nonreturn rate: Apply of suppression-subtractive hybridization to identify functional markers for fertility. Biol. Reprod. 78, 618–635. https://doi.org/10.1095/biolreprod.106.059030 (2008).
-
Lalancette, C., Faure, R. L. & Leclerc, P. Identification of the proteins present in the bull sperm cytosolic fraction enriched in tyrosine kinase activity: a proteomic approach. Proteomics 6, 4523–4540. https://doi.org/10.1002/pmic.200500578 (2006).
-
Byrne, Yard., Leahy, T., McCulloch, R., Colgrave, M. L. & Kingdom of the netherlands, M. Thou. Comprehensive mapping of the bull sperm surface proteome. Proteomics 12, 23–24. https://doi.org/10.1002/pmic.201200133 (2012).
-
Dawe, H. R., Farr, H., Portman, Due north., Shaw, Thou. G. & Gull, K. The Parkin co-regulated gene product, PACRG, is an evolutionarily conserved axonemal protein that functions in outer-doublet microtubule morphogenesis. J. Prison cell Sci. 118, 5421–5430. https://doi.org/10.1242/jcs.02659 (2005).
-
Lehti, M. S. & Sironen, A. Formation and function of sperm tail structures in association with sperm motion defects. Biol. Reprod. 97, 522–536. https://doi.org/10.1093/biolre/iox096 (2017).
-
Guo, F. et al. Alternative splicing, promotor, methylation, and functional SNPs of sperm flagella ii gene in testis and mature spermatozoa of Holstein bulls. Reprod. 147, 241–252. https://doi.org/ten.1530/REP-thirteen-0343 (2014).
-
Pratt, S. L. et al. Identification of bovine prolactin in seminal fluid, and expression and localization of the prolactin receptor and prolactin-inducible protein in the testis and epididymis of bulls exposed to ergot alkaloids. Theriogenology https://doi.org/10.1016/j.theriogenology.2014.10.031 (2014).
-
Huang, Due west., Mikhail, D., Bindrim, A. C. & Khatib, H. Interactions of the bovine placental lactogen and prolactin receptor genes are associated with fertility traits in cattle. Anim. three, 1743–1745. https://doi.org/10.1017/S1751731109990826 (2009).
-
Jaillard, South. et al. Assortment-CGH diagnosis in ovarian failure: identification of new molecular actors for ovarian physiology. J. Ovarian Res. https://doi.org/ten.1186/s13048-016-0272-five (2016).
-
Whittington, C. M. et al. Transcriptomic changes in the pre-implantation uterus highlight histotrophic diet of the developing marsupial embryo. Sci. Rep. https://doi.org/10.1038/s41598-018-20744-z (2018).
-
Coyral-Castel, S. et al. KIRREL is differentially expressed in adipose tissue from 'fertil+' and 'fertil−' cows: in vitro office in ovary?. Reprod. 155, 181–196. https://doi.org/10.1530/REP-17-0649 (2018).
-
Cochran, South. D., Cole, J. B., Null, D. J. & Hansen, P. J. Discovery of single nucleotide polymorphisms in candidate genes associated with fertility and product traits in Holstein cattle. BMC Genet. https://doi.org/10.1186/1471-2156-fourteen-49 (2013).
-
Tonellotto dos Santos, J. et al. Molecular characterization and regulation of the angiotensin-converting enzyme type ii/Angiotensin-(1–7)/MAS receptor axis during the ovulation process in cattle. J. Renin-Angio-Aldo. S. xiii, 91–98. https://doi.org/ten.1177/1470320311417273(2011).
-
Li, K. W. K., Mruk, D. D. & Cheng, C. Y. Mitogen-activated poly peptide kinases in male person reproductive function. Trends Mol. Med. fifteen, 159–168. https://doi.org/ten.1016/j.molmed.2009.02.002 (2009).
-
Dun, M. D., Aitken, R. J. & Nixon, B. The part of molecular chaperons in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum. Reprod. Update 18, 420–435. https://doi.org/10.1093/humupd/dms009 (2012).
-
Howes, E. A., Hurst, S. M. & Jones, R. Actin and actin-binding proteins in bovine spermatozoa: Potential role in membrane remodeling and intracellular signaling during epididymal maturation and the acrosome reaction. J. Androl. 22, 62–72 (2001).
-
Staub, C. & Johnson, 50. Review: Spematogensis in the bull. Animate being 12, 27–35. https://doi.org/10.1017/S1751731118000435 (2018).
-
Palasz, A. T., Cates, Westward. F., Barth, A. D. & Mapletoft, R. J. The relationship between scrotal circumference and quantitative testicular traits in yearling beefiness bulls. Theriogenology 42, 715–726. https://doi.org/10.1016/0093-691X(94)90388-Y (1994).
-
Kim, J. H., Jee, B. C., Lee, J. M., Suh, C. S. & Kim, S. H. Histone acetylation level and histone acetyltransferase/deacetylase action in ejaculated sperm from normozoospermic men. Yonsei Med. J. 55, 1333–1340. https://doi.org/10.3349/ymj.2014.55.5.1333 (2014).
-
Kerns, One thousand., Zigo, M. & Sutovsky, P. Zinc: A necessary ion for mammalian sperm fertilization competency. Int. J. Mol. Sci. 19, 4097. https://doi.org/10.3390/ijms19124097 (2018).
-
Santi, C. M., Orta, Thou., Visconti, P. E., Darszon, A. & Treviño, C. Fifty. Grand+ and Cl− channels and transporters in sperm office. Curr. Meridian. Dev. Biol. 102, 385–421. https://doi.org/x.1016/B978-0-12-416024-8.00014-3 (2013).
-
Ma, D., Wang, D. & Yang, Westward. Kinesins in spermatogenesis. Biol. Reprod. 96, 267–276. https://doi.org/ten.1095/biolreprod.116.144113 (2017).
-
Schenkel, F. Southward., Miller, S. P. & Wilton, J. W. Genetic parameters and breed differences for feed efficiency, growth, and trunk composition traits of young beefiness bulls. Can. J. Anim. Sci. 84, 177–218. https://doi.org/10.4141/A03-085 (2003).
-
Devkota, B. et al. Relationships among age, body weight, scrotal circumference, semen quality, and peripheral testosterone, and estradiol concentrations in pubertal and postpubertal Holstein bulls. J. Vet. Med. Sci. seventy, 119–121 (2008).
-
Trocóniz, J. F., Beltrán, J., Bastidas, H., Larreal, H. & Bastidas, P. Testicular development, body weight changes, puberty and semen traits of growing Guzerat and Nellore bulls. Theriogenology 35, 815–826. https://doi.org/10.1016/0093-691X(91)90422-A (1991).
-
Bolormaa, Southward. et al. A multi-trait, meta-analysis for detecting pleiotropic polymorphisms for stature, fatness and reproduction in beefiness cattle. PloS Genet. ten, e1004198. https://doi.org/10.1371/periodical.pgen.1004198 (2014).
-
Fonseca, P. A. Due south., Suárez-Vega, A. & Cánovas, A. Weighted gene correlation network meta-assay reveals functional candidate genes associated with loftier-and sub-fertile reproductive functioning in beef cattle. Genes 11, 543 (2020).
-
Paputungan, U. & Makarechian, M. The influence of dam weight, body status and udder scores on dogie birth weight and preweaning growth rates in beefiness cattle. Asian Austral. J. Anim. Sci. 13, 435–439. https://doi.org/x.5713/ajas.2000.435 (2000).
-
Devani, K., Valente, T. S., Crowley, J. J. & Orsel, G. Development of optimal genetic evaluations for teat and udder structure in Canadian Angus cattle. J. Anim. Sci. https://doi.org/10.1093/jas/skz314 (2019).
-
Johnston, D. J., Corbet, N. J., Barwick, S. A., Wolcott, G. L. & Holroyd, R. One thousand. Genetic correlations of immature bull reproductive traits and heifer puberty traits with female person reproductive functioning in two tropical beef genotypes in northern Australia. Anim. Prod. Sci. 54, 74–84. https://doi.org/10.1071/AN13044 (2014).
-
Gargantini, Grand., Cundiff, Fifty. 5., Lunstra, D. D. & Van Vleck, L. D. Genetic relationships betwixt male and female person reproductive traits in beef cattle. Prof. Anim. Sci. 21, 195–199 (2005).
-
McClure, K. C. et al. A genome browse for quantitative trait loci influencing carcass, mail service-natal growth and reproductive traits in commercial Angus cattle. Anim. Genet. 41, 597–607. https://doi.org/10.1111/j.1365-2052.2010.02063.ten (2010).
-
Pursel, V. G. & Johnson, L. A. Freezing of boar spermatozoa: fertilizing chapters with concentrated semen and a new thawing procedure. J. Anim. Sci. 40, 99–102. https://doi.org/x.2527/jas1975.40199x (1975).
-
Awada, B. J., Mackenzie-Bell, K. & Buhr, M. Thousand. Reactive oxygen boar species and boar sperm office. Biol. Reprod. 81, 553–561. https://doi.org/10.1095/biolreprod.109.076471 (2009).
-
Purcell, Southward. et al. PLINK: A tool ready for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. https://doi.org/ten.1086/519795 (2007).
-
Misztal, I. et al. BLUPF90 family of programs (2014).
-
de Oliveira Fragomeni, B. et al. Changes in variance explained past summit SNP windows over generations for three traits in broiler chicken. Front. Genet. https://doi.org/10.3389/fgene.2014.00332 (2014).
-
Asselstine, 5. et al. Genetic mechanisms regulating the host response during mastitis. J. Dairy Sci. 102, 9043–9059. https://doi.org/x.3168/jds.2019-16504 (2019).
-
Hu, Z., Park, C. A., Wu, X. & Reecy, J. M. Creature QTLdb: an improved database tool for livestock creature QTL/association data dissemination in the postal service-genome era. Nucleic Acids Res. 41, D871–D879. https://doi.org/10.1093/nar/gks1150 (2013).
-
Chen, J., Bardes, E. E., Aronow, B. J. & Jegga, A. 1000. Toppgene suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311. https://doi.org/10.1093/nar/gkp427 (2009).
-
Guney, Due east., Garcia-Garcia, J. & Oliva, B. Guildify: a web server for phenotypic label of genes through biological data integration and network-based prioritization algorithms. Bioinform. 30, 1789–1790. https://doi.org/ten.1093/bioinformatics/btu092 (2014).
-
Xia, J., Gill, E. E. & Hancock, R. E. NetworkAnalyst for statistical, visual and network-based meta-assay of gene expression data. Nat. Protoc. x, 823. https://doi.org/10.1038/nprot.2015.052 (2015).
-
Cánovas, A. et al. Segregation of regulatory polymorphisms with furnishings on the gluteus medius transcriptome in a purebred grunter population. PLoS Ane vii, e35583. https://doi.org/10.1371/journal.pone.0035583 (2012).
Acknowledgements
The authors acknowledge financial support from the (FDE.13.17) Sustainable Beef and Forage Science Cluster funded by the Canadian Beef Cattle Check-Off, Beef Cattle Research Quango (BCRC), Alberta Beef Producers, Alberta Cattle Feeders' Clan, Beefiness Farmers of Ontario, La Fédération des Productuers de bovins du Québec, and Agriculture and Agri-Food Canada's Canadian Agricultural Partnership. This written report was also supported by the Ontario Ministry building of Agriculture, Nutrient, and Rural Affairs (OMAFRA), Ontario Ministry of Research and Innovation, Agriculture and Agri-Nutrient Canada (AAFC), and Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant. Hannah Sweett was supported by the OMAFRA Highly Qualified Personnel Scholarship Program.
Author data
Authors and Affiliations
Contributions
A.C. was responsible for the conceptualization, experimental design, and theoretical discussions. P.A.S.F., A.S.-Five., and A.L. were responsible for data curation. A.C., H.S. and P.A.S.F. conducted the GWAS, cistron note, QTL notation and functional analysis. A.C. was responsible for funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Boosted information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Artistic Eatables Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you lot requite appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables licence, and betoken if changes were fabricated. The images or other third party cloth in this commodity are included in the article'south Creative Eatables licence, unless indicated otherwise in a credit line to the textile. If cloth is non included in the commodity's Creative Eatables licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you volition need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/past/4.0/.
Reprints and Permissions
Near this article
Cite this article
Sweett, H., Fonseca, P.A.Southward., Suárez-Vega, A. et al. Genome-wide association study to place genomic regions and positional candidate genes associated with male fertility in beefiness cattle. Sci Rep ten, 20102 (2020). https://doi.org/10.1038/s41598-020-75758-3
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-020-75758-three
Farther reading
Comments
By submitting a comment yous concur to abide by our Terms and Community Guidelines. If you detect something calumniating or that does not comply with our terms or guidelines delight flag it as inappropriate.
Source: https://www.nature.com/articles/s41598-020-75758-3
Post a Comment for "Fertile Male Dairy Cattle Fertile Male Beef Cattle"